An Industry Poised for Growth
Exploring E-beam and X-ray:
An Industry Poised for Growth
The sterilization industry stands at a crossroads, driven by growing concerns surrounding the environmental and health impacts of ethylene oxide (EtO) sterilization, a long-dominant method in the sector.
The global sterilization services market relies heavily on EtO, which accounts for approximately 50% of sterilization processes. However, EtO's classification as a carcinogen and increasingly stringent emissions regulations have cast a shadow on its future, creating a significant opportunity for other technologies, such as e-beam and x-ray sterilization, to emerge as viable and sustainable solutions.
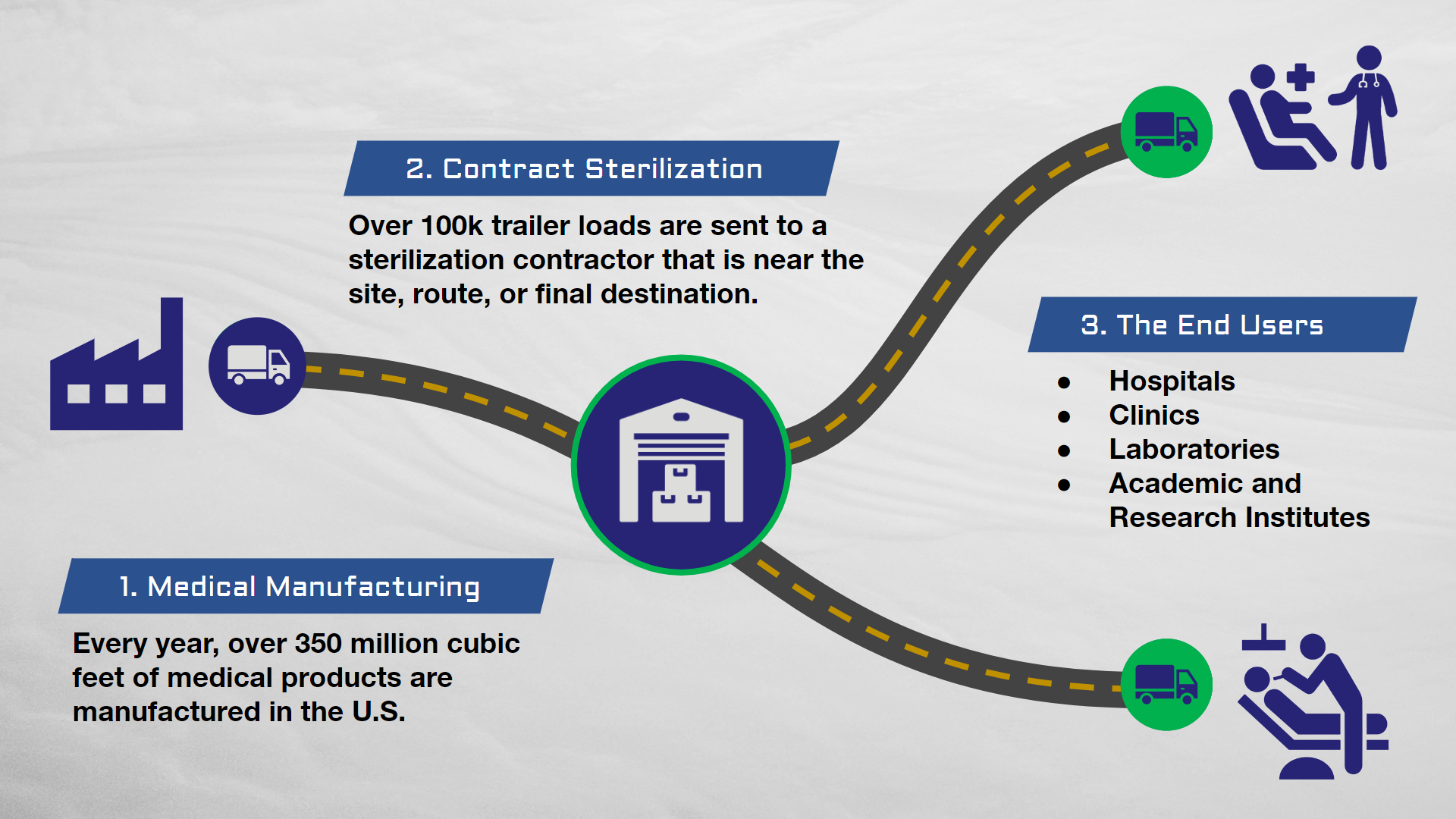
A Wake-Up Call for the Industry
The closure of the Sterigenics Willowbrook sterilization facility in 2019 served as a stark wake-up call for the medical device industry. The plant, a major provider of ethylene oxide (EtO) sterilization services, was shut down due to concerns over elevated EtO emissions into the surrounding community. This event exposed the vulnerabilities of overreliance on EtO sterilization and triggered a domino effect, leading to capacity constraints, increased costs, and logistical challenges for medical device manufacturers. The facility closure ultimately underscored the importance of exploring and adopting alternative sterilization methods to ensure the continued availability of safe and effective medical devices.
A World without EO: The Rise of E-beam and X-ray
Gamma sterilization dominates the remainder of the market, but expanding this technology poses several challenges beyond just constructing new facilities. The sourcing of Cobalt-60, the essential radioactive isotope, is limited to a few global suppliers. Moreover, dependence on nuclear reactors for Cobalt-60 production adds complexity and cost, while stringent regulations govern its handling and disposal. These factors impede scalability and underscore the need for machine-based sterilization methods.
In a hypothetical scenario where EtO sterilization is phased out, the demand for alternative methods would surge dramatically. E-beam and x-ray sterilization, with their established efficacy and environmental advantages, are well-positioned to fill this gap.
- E-beam Sterilization:
This method utilizes accelerated electrons to effectively eliminate microorganisms. It offers advantages such as rapid processing times, low operating costs, and compatibility with a wide range of materials. - X-ray Sterilization:
X-ray sterilization employs high-energy X-rays to achieve sterilization. It boasts excellent penetration capabilities, making it suitable for dense or complex products, and offers a superior dose uniformity ratio compared to gamma irradiation.

Meeting the Demand: Building New Infrastructure
Transitioning away from EtO sterilization requires significantly expanding e-beam and x-ray capabilities, necessitating a strategic approach to building new infrastructure. This involves constructing new sterilization plants equipped with e-beam and x-ray technology strategically located across geographical regions to effectively meet the anticipated growth in demand.
E-beam offers high efficiency for products with lower densities, while X-ray can handle products with higher densities that are beyond the reach of e-beam. This combination ensures that the industry has the tools to sterilize a wide range of medical devices effectively and efficiently.
The Future of Sterilization
The shift away from EtO presents both challenges and opportunities for the sterilization industry. E-beam and x-ray sterilization technologies are on the brink of significant growth, driven by their effectiveness, environmental benefits, and the increasing demand for safe and sustainable sterilization solutions. By investing in infrastructure, technology, and collaboration, the industry can ensure a smooth transition and meet the evolving needs of the healthcare sector and beyond.
Click here for more insight on the current and future trends of e-beam and x-ray sterilization technology, including resources for your current irradiation process.



